💬 Introduction
This chapter name is chemical kinetics class 12 notes pdf.
Have you ever wondered why milk sours faster in summer or why a rusted nail takes months to form? 🤔
That’s Chemical Kinetics in action — the science of how fast reactions happen and what makes them faster or slower.
If you’re a Class 12 student preparing for Boards, JEE, or NEET, this topic is pure gold! ✨
Here you’ll find beautiful handwritten notes, key formulas, and crystal-clear explanations — all aligned with NCERT.
📥 Download Handwritten Notes PDF: (Scrol down)
👉 Click Here to Download Chemical Kinetics Class 12 Handwritten Notes (Free)
⚗️ What is Chemical Kinetics? (chemical kinetics handwritten notes class 12)
In simple words, Chemical Kinetics = Speed of a Chemical Reaction.
It tells us:
- How quickly reactants turn into products 🔄
- What affects that speed (temperature, concentration, catalyst, etc.)
- How to calculate the rate mathematically
Example:
When magnesium reacts with HCl, bubbles appear instantly.
But when iron rusts, it takes days.
👉 Both are chemical reactions — but with different rates.
🧭 Rate of Reaction — The Heart of Kinetics
The rate of a chemical reaction is defined as:
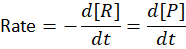
This simply means how much reactant decreases or how much product increases per second.
💡 Fast reaction: Explosion of hydrogen gas
🐢 Slow reaction: Rusting of iron
⚙️ Factors Affecting Rate of Reaction
| Factor | How It Affects Rate | Example |
| Concentration | More molecules = more collisions | Dilute acid reacts slower than concentrated acid |
| Temperature | Higher T → faster reaction | Food spoils faster in heat |
| Catalyst | Speeds up reaction without being used | Enzymes in our body |
| Surface Area | Finer particles react faster | Powdered zinc reacts faster than zinc strip |
| Nature of Reactants | Ionic reactions are faster than covalent | NaCl + AgNO₃ is instant |
🧮 Rate Law & Rate Constant (k)
For a general reaction:
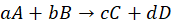
Rate =
Here:
- x, y = order of reaction (found experimentally)
- k = rate constant (depends on temperature, not concentration)
| Order | Unit of k | Example |
| Zero | mol L⁻¹ s⁻¹ | Photochemical reaction |
| First | s⁻¹ | Decomposition of N₂O₅ |
| Second | L mol⁻¹ s⁻¹ | Reaction between NO and Cl₂ |

⏱️ Integrated Rate Equations
🥇 First Order Reaction
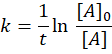
👉 Half-Life:
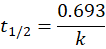
Constant — doesn’t depend on [A].
🥈 Second Order Reaction
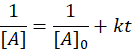
👉 Half-Life:
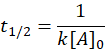
Decreases with increasing concentration. (chemical kinetics class 12 ncert pdf)
🔥 Temperature Dependence — Arrhenius Equation
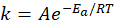
Where:
- Ea = activation energy
- R = gas constant (8.314 J/mol·K)
- T = temperature in Kelvin
➡️ Higher temperature → higher k → faster reaction.
📊 Graph Tip:
ln k vs 1/T gives a straight line with slope = −Ea/R.
🧩 Catalyst – The Reaction Accelerator
A catalyst provides a new path with lower activation energy.
Think of it like a shortcut in a traffic jam 🚗💨 — you reach faster without changing your destination!
✅ Catalyst does not change:
- ΔH (enthalpy)
- Final composition of products
📘 Order vs Molecularity (Easy Table) (chemical kinetics formulas class 12)
| Property | Order | Molecularity |
| Definition | Sum of powers in rate law | Number of molecules in a single step |
| Value | Can be 0, 1, 2, fractional | Always whole number |
| Type | Experimental | Theoretical |
| Example | Rate = k[A]² → 2nd order |
🧾 Quick Formula Sheet (Must-Memorize)
| Concept | Formula |
| Rate Law | |
| Half-life (1st order) | |
| Arrhenius Equation | |
| Integrated Rate (1st order) | |
| Second Order |
✍️ Why You’ll Love These Handwritten Notes
✅ Clean, exam-oriented handwriting
✅ Covers all NCERT derivations & graphs
✅ Includes short tricks for numericals
✅ Perfect for last-minute revision
✅ Helpful for Boards, JEE, NEET
📥 Download the Handwritten PDF Now
Masternotes – Homepage
💬 FAQs — Chemical kinetics class 12 ncert pdf
Q1. What is the best definition of Chemical Kinetics?
It’s the study of the rate of chemical reactions and the factors affecting them. chemical kinetics class 12 ncert pdf download now.
Q2. Is this topic important for boards?
Absolutely — it carries 4 to 6 marks in CBSE exams every year.
Q3. What is the order of a reaction?
It’s the sum of powers of reactant concentrations in the rate law.
Q4. How can I score full marks in this chapter?
Memorize key formulas, practice NCERT numericals, and revise using these handwritten notes.
Q5. What is activation energy?
The minimum energy required by reactants to form products. class 12 chemistry notes pdf Downlaod now.
🚀 Final Revision Tips :- chemical kinetics handwritten notes class 12
🔹 Practice at least 10 numericals daily
🔹 Revise graphs (ln k vs 1/T, [A] vs t)
🔹 Focus on differences (Order vs Molecularity)
🔹 Use your handwritten notes 1 day before exam — quick recall guaranteed! 💯
class 12 chemistry notes pdf
Thanks For Your support.
About Author
Name – Ankita
Teacher Name – Dinesh Kumar
| S.NO | Name of Chapter | View |
| 1. | Electric Charges and Fields | Click |
| 2. | Electrostatic Potential and Capacitance | Click |
| 3. | Current Electricity | Click |
| 4. | Moving Charges and Magnetism | Click |
| 5. | Magnetism and Matter | Click |
| 6. | Electromagnetic Induction | Click |
| 7. | Alternating Current | Click |
| 8. | Electromagnetic Waves | Click |
| 9. | Ray Optics and Optical Instruments | Click |
| 10. | Wave Optics | Click |
| 11. | Dual Nature of Radiation and Matter | Click |
| 12. | Atoms | Click |
| 13. | Nuclei | Click |
| 14. | Semiconductor Electronics | Click |
| 15. | Communication Systems | Click |
| THANKU |
Comprehensive Coverage: These notes provide an in-depth analysis of each topic, covering all essential concepts in coordination compounds.
Easy-to-Understand Language: The notes are written in a clear and concise manner, making complex ideas easier to grasp.
Illustrative Diagrams: Visual aids and diagrams accompany the explanations, facilitating better understanding and retention.
Exam-Oriented: Designed with Class 12 board exams in mind, these notes are tailored to help you excel in your examinations.
Whether you are a student aiming for top grades or someone curious about the fascinating world of coordination compounds, these master notes are your ultimate resource. Enhance your knowledge, strengthen your concepts, and unlock the secrets of coordination compounds with this all-encompassing study material. Happy learning! Youtube







